Project C5
Influence of gut microbiota on Candida albicans colonization, host immune response and candidiasis
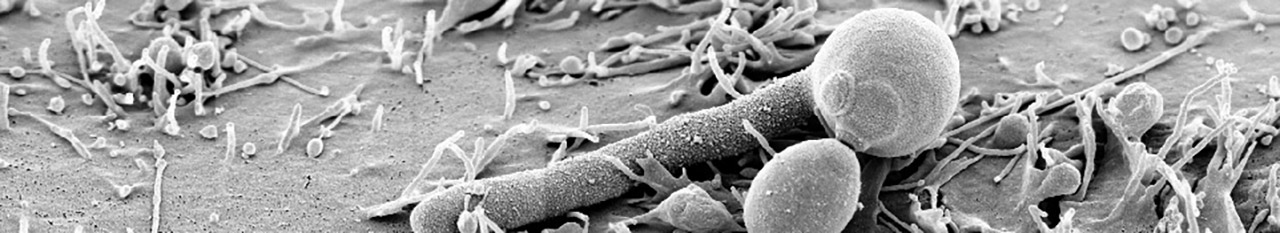
Increasing evidence suggests a critical role of the microbiota for development of infections, mediated by both direct interactions between microorganisms and indirectly by modulation of immune responses. We hypothesize that the balance between microbiota, C. albicans and the host defense mechanisms affects the development of disseminated candidiasis. C5 will therefore address the role of the microbiota for (i) colonization resistance towards C. albicans, (ii) immune responses during colonization and (iii) the role of microbiota for susceptibility to systemic candidiasis.
Therefore, we will use genetically identical mice from different breeding facilities that can be expected to harbor distinct microbiomes. The functional consequences of different microbiota composition will be initially assessed determining the colonization resistance of the mice to C. albicans. Based on this, the host response to C. albicans colonization will be analyzed in detail in mice with different microbiota composition to deduce to which extend the existing microbiota affects the immunological response to C. albicans. Furthermore, we will determine to which extent differences in microbiota composition affect the clinical outcome of translocation and systemic candidiasis. In parallel microbiome and mycobiota analysis will be performed in human patients to analyze if patterns observed in mice are transferable to human patients.

Prof. Dr. Ilse D. Jacobsen
Department of Microbial Immunology
Friedrich Schiller University Jena
Research Group Microbial Immunology
Leibniz Institute for Natural Product Research and Infection Biology - Hans-Knöll-Institute

Prof. Dr. Berit Jungnickel
Center for Molecular Biomedicine
Institute of Biochemistry and Biophysics
Friedrich Schiller University Jena
| Author | Year | Title | Journal | Links |
|---|---|---|---|---|
| Böttcher B, Kienast SD, Leufken J, Eggers C, Sharma P, Leufken CM, Morgner B, Drexler HCA, Schulz D, Allert S, Jacobsen ID, Vylkova S, Leidel SA, Brunke S | 2024 | A highly conserved tRNA modification contributes to C. albicans filamentation and virulence. | Microbiol Spectr. e0425522 | PubMed |
| Jacobsen, I.D. | 2023 | The Role of Host and Fungal Factors in the Commensal-to-Pathogen Transition of Candida albicans. | Curr Clin Micro Rpt: 23-190-w | Curr Clin Micro Rpt |
| Langenhorst D, Fürst AL, Alberter K, Vilhena C, Dasari P, Daud M, Heilig L, Luther CH, Dittrich M, Reiher N, Wich M, Elmowafy M, Jacobsen ID, Jungnickel B, Zipfel PF, Beyersdorf N. | 2023 | Soluble Enolase 1 of Candida albicans and Aspergillus fumigatus Stimulates Human and Mouse B Cells and Monocytes. | J Immunol. 12: ji2200318 | PubMed |
| Rebai, Y.; Wagner, L.; Gnaien, M.; Hammer, M.L.; Kapitan, M.; Niemiec, M.J.; Mami, W.; Mosbah, A.; Messadi, E.; Mardassi, Vylkova S, Jacobsen ID, Znaidi S. | 2023 | Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage. | Microorganisms 11, 1929 | Microorganisms |
| Halder LD, Babych S, Palme DI, Mansouri-Ghahnavieh E, Ivanov L, Ashonibare V, Langenhorst D, Prusty B, Rambach G, Wich M, Trinks N, Blango MG, Kornitzer D, Terpitz U, Speth C, Jungnickel B, Beyersdorf N, Zipfel PF, Brakhage AA, Skerka C | 2022 | Candida albicans induces cross-kingdom miRNA trafficking in human monocytes to promote fungal growth. | mBio 13(1): e0356321 | mBio |
| Ramírez-Zavala B, Krüger I, Dunker C, Jacobsen ID, Morschhäuser J | 2022 | The protein kinase Ire1 has a Hac1-independent essential role in iron uptake and virulence of Candida albicans. | PLoS Pathog 18(2): e1010283 | PubMed |
| Niemiec MJ, Kapitan M, Himmel M, Döll K, Krüger T, Köllner TG, Auge I, Kage F, Alteri CJ, Mobley HLT, Monsen T, Linde S, Nietzsche S, Kniemeyer O, Brakhage AA, Jacobsen ID | 2022 | Augmented Enterocyte Damage During Candida albicans and Proteus mirabilis Coinfection. | Front Cell Infect Microbiol. 12:866416 | PubMed |
| Jungnickel B, Jacobsen ID | 2022 | Systemic candidiasis in mice: new insights from an old model. | Front Fungal Biol 3: 940884 | Front Fungal Biol |
| Montaño DE, Hartung S, Wich M, Ali R, Jungnickel B, von Lilienfeld-Toal M, Voigt K | 2022 | The TLR-NF-kB axis contributes to the monocytic inflammatory response against a virulent strain of Lichtheimia corymbifera, a causative agent of invasive mucormycosis. | Front Immunol. 13: 882921 | PubMed |
| Machata S, Sreekantapuram S, Hünniger K, Kurzai O, Dunker C, Schubert K, Krüger W, Schulze-Richter B, Speth C, Rambach G, Jacobsen ID | 2021 | Significant differences in host-pathogen interactions between murine and human whole blood. | Front Immunol 11: 3489 | Frontiers |
| Dunker C, Polke M, Schulze-Richter B, Schubert K, Rudolphi S, Gressler AE, Pawlik T, Prada Salcedo JP, Niemiec MJ, Slesiona-Künzel S, Swidergall M, Martin R, Dandekar T, Jacobsen ID | 2021 | Rapid proliferation due to better metabolic adaptation results in full virulence of a filament-deficient Candida albicans strain. | Nat Commun 12: 3899 | PubMed |
| Ferreira-Gomes M, Wich M, Böde S, Hube B, Jacobsen ID, Jungnickel B | 2021 | B cell recognition of Candida albicans hyphae via TLR 2 promotes IgG1 and IL-6 Secretion for TH17 differentiation. | Front Immunol 12: 698849 | PubMed |
| Wich M, Greim S, Ferreira-Gomes M, Krüger T, Kniemeyer O, Brakhage AA, Jacobsen ID, Hube B, Jungnickel B | 2021 | Functionality of the human antibody response to Candida albicans. | Virulence 12: 3137-48 | PubMed |
| Böttcher K, Braunschmidt K, Hirth G, Schärich K, Klassert TE, Stock M, Sorgatz J, Fischer-Burkart S, Ullrich S, Frankenberger S, Kritsch D, Kosan C, Küppers R, Strobl LJ, Slevogt H, Zimber-Strobl U, Jungnickel B | 2021 | Context-dependent regulation of immunoglobulin mutagenesis by p53. | Molecular Immunology 138: 128–136 | PubMed |
| Halder LD, Jo EAH, Hasan MZ, Ferreira-Gomes M, Krüger T, Westermann M, Palme DI, Rambach G, Beyersdorf N, Speth C, Jacobsen ID, Kniemeyer O, Jungnickel B, Zipfel PF, Skerka C | 2020 | Immune modulation by complement receptor 3 dependent human monocyte TGF-β1-transporting vesicles. | Nat Commun 11: 2331 | PubMed |
| Ruben S, Garbe E, Mogavero S, Albrecht-Eckardt D, Hellwig D, Häder A, Krüger T, Gerth K, Jacobsen ID, Elshafee O, Brunke S, Hünniger K, Kniemeyer O, Brakhage AA, Morschhäuser J, Hube B, Vylkova S, Kurzai O, Martin R | 2020 | Ahr1 and Tup1 contribute to the transcriptional control of virulence-associated genes in Candida albicans. | mBio 11: e00206-20 | PubMed |
| Gerwien F, Dunker C, Brandt P, Garbe E, Jacobsen ID, Vylkova S | 2020 | Clinical Candida albicans vaginal isolates and a laboratory strain show divergent behaviors during macrophage interactions. | mSphere 5: e00393-20 | PubMed |
| Arend N, Pittner A, Ramoji A, Mondol AS, Dahms M, Rüger J, Kurzai O, Schie IW, Bauer M, Popp J, Neugebauer U | 2020 | Detection and differentiation of bacterial and fungal Infection of neutrophils from peripheral blood using raman spectroscopy. | Anal Chem 92: 10560-8 | PubMed |
| Machata S, Müller MM, Lehmann R, Sieber S, Panagiotou G, Carvalho A, Cunha C, Lagrou K, Maertens J, Slevogt H, Jacobsen ID | 2020 | Proteome analysis of bronchoalveolar lavage fluids reveals host and fungal proteins highly expressed during invasive pulmonary aspergillosis in mice and humans. | Virulence 11: 1337-51 | Virulence |
| Khaliq W, Großmann P, Neugebauer S, Kleyman A, Domizi R, Calcinaro S, Brealey D, Gräler M, Kiehntopf M, Schäuble S, Singer M, Panagiotou G, Bauer M | 2020 | Lipid metabolic signatures deviate in sepsis survivors compared to non-survivors. | Comput Struct Biotechnol J 18: 3678-3691 | Science direct |
| Matthias J, Heink S, Picard F, Zeiträg J, Kolz A, Chao YY, Soll D, de Almeida GP, Glasmacher E, Jacobsen ID, Riedel T, Peters A, Floess S, Huehn J, Baumjohann D, Huber M, Korn T, Zielinski CE | 2020 | Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. | J Clin Invest 130: 4587-600 | PubMed |
| von Müller C, Bulman F, Wagner L, Rosenberger D, Marolda A, Kurzai O, Eißmann P, Jacobsen ID, Perner B, Hemmerich P, Vylkova S | 2020 | Active neutrophil responses counteract Candida albicans burn wound infection of ex vivo human skin explants. | Sci Rep 10: 21818 | PubMed |
| Krüger W, Vielreicher S, Kapitan M, Jacobsen ID, Niemiec MJ | 2019 | Fungal-bacterial interactions in health and disease. | Pathogens 8: 70 (Review) | PubMed |
| Klaile E, Müller MM, Brehme S, Zubiria-Barrera C, Klassert TE, Stock M, Durotin A, Nguyen TD, Feer S, Singer BB, Zipfel PF, Rudolphi S, Jacobsen ID, Slevogt H | 2019 | Unaltered fungal burden and lethality in human CEACAM1-transgenic mice during Candida albicans dissemination and systemic infection. | Front Microbiol 10: 2703 | PubMed |
| Maurer M, Gresnigt MS, Last A, Wollny T, Berlinghof F, Pospich R, Cseresnyes Z, Medyukhina A, Graf K, Gröger M, Raasch M, Siwczak F, Nietzsche S, Jacobsen ID, Figge MT, Hube B, Huber O, Mosig AS | 2019 | A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. | Biomaterials 220: 119396 | PubMed |
| Polke M, Leonhardt I, Kurzai O, Jacobsen ID | 2018 | Farnesol signalling in Candida albicans - more than just communication. | Crit Rev Microbiol 44: 230-43 (Review) | PubMed |
| Schaarschmidt B, Vlaic S, Medyukhina A, Neugebauer S, Nietzsche S, Gonnert FA, Rödel J, Singer M, Kiehntopf M, Figge MT, Jacobsen ID, Bauer M, Press AT | 2018 | Molecular signatures of liver dysfunction are distinct in fungal and bacterial infections in mice. | Theranostics 8: 3766-80 | PubMed |
| Irmscher S, Döring N, Halder LD, Jo EAH, Kopka I, Dunker C, Jacobsen ID, Luo S, Slevogt H, Lorkowski S, Beyersdorf N, Zipfel PF, Skerka C | 2018 | Kallikrein cleaves C3 and activates complement. | J Innate Immun 10: 94-105 | PubMed |
| Allert S*, Förster TM*, Svensson CM, Richardson JP, Pawlik T, Hebecker B, Rudolphi S, Juraschitz M, Schaller M, Blagojevic M, Morschhäuser J, Figge MT, Jacobsen ID, Naglik JR, Kasper L, Mogavero S, Hube B; *authors contributed equally | 2018 | Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. | mBio 9: e00915-18 | PubMed |
| Conrad T, Kniemeyer O, Henkel SG, Krüger T, Mattern DJ, Valiante V, Guthke R, Jacobsen ID, Brakhage AA, Vlaic S, Linde J | 2018 | Module-detection approaches for the integration of multilevel omics data highlight the comprehensive response of Aspergillus fumigatus to caspofungin. | BMC Syst Biol 12: 88 | PubMed |
| Luo S, Dasari P, Reiher N, Hartmann A, Jacksch S, Wende E, Barz D, Niemiec MJ, Jacobsen I, Beyersdorf N, Hünig T, Klos A, Skerka C, Zipfel PF | 2018 | The secreted Candida albicans protein Pra1 disrupts host defense by broadly targeting and blocking complement C3 and C3 activation fragments. | Mol Immunol 93: 266-77 | PubMed |
| Polke M, Sprenger M, Scherlach K, Albán-Proaño MC, Martin R, Hertweck C, Hube B, Jacobsen ID | 2017 | A functional link between hyphal maintenance and quorum sensing in Candida albicans. | Mol Microbiol 103: 595-617 | PubMed |
| Halder LD, Abdelfatah MA, Jo E, Jacobsen ID, Westermann M, Beyersdorf N, Lorkowski S, Zipfel PF, Skerka C | 2017 | Factor H binds to extracellular DNA traps released from human blood monocytes in response to Candida albicans. | Front Immunol 7: 671 | PubMed |
| Schulze B, Rambach G, Schwartze VU, Voigt K, Schubert K, Speth C, Jacobsen ID | 2017 | Ketoacidosis alone does not predispose to mucormycosis by Lichtheimia in a murine pulmonary infection model. | Virulence 8: 1657-67 | PubMed |
| Jacobsen ID, Hube B | 2017 | Candida albicans morphology: still in focus. | Expert Rev Anti Infect Ther 15: 327-30 (Editorial) | PubMed |
| Kroll K, Shekhova E, Mattern DJ, Thywissen A, Jacobsen ID, Strassburger M, Heinekamp T, Shelest E, Brakhage AA, Kniemeyer O | 2016 | The hypoxia-induced dehydrogenase HorA is required for coenzyme Q10 biosynthesis, azole sensitivity and virulence of Aspergillus fumigatus. | Mol Microbiol 101: 92-108 | PubMed |
| Hebecker B, Vlaic S, Conrad T, Bauer M, Brunke S, Kapitan M, Linde J, Hube B, Jacobsen ID | 2016 | Dual-species transcriptional profiling during systemic candidiasis reveals organ-specific host-pathogen interactions. | Sci Rep 6: 36055 | PubMed |
| Luo T, Krüger T, Knüpfer U, Kasper L, Wielsch N, Hube B, Kortgen A, Bauer M, Giamarellos-Bourboulis EJ, Dimopoulos G, Brakhage AA, Kniemeyer O | 2016 | Immunoproteomic analysis of antibody responses to extracellular proteins of Candida albicans revealing the importance of glycosylation for antigen recognition. | J Proteome Res 15: 2394-406 | PubMed |
| Förster TM, Mogavero S, Dräger A, Graf K, Polke M, Jacobsen ID, Hube B | 2016 | Enemies and brothers in arms: Candida albicans and gram-positive bacteria. | Cell Microbiol 18: 1709-15 | PubMed |
| Duggan S, Essig F, Hünniger K, Mokhtari Z, Bauer L, Lehnert T, Brandes S, Häder A, Jacobsen ID, Martin R, Figge MT, Kurzai O | 2015 | Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. | Cell Microbiol 17: 1259-76 | PubMed |
| Polke M, Hube B, Jacobsen ID | 2015 | Candida survival strategies. | Adv Appl Microbiol 91: 139-235 | PubMed |
| Jacobsen ID, Lüttich A, Kurzai O, Hube B, Brock M | 2014 | In vivo imaging of disseminated murine Candida albicans infection reveals unexpected host sites of fungal persistence during antifungal therapy. | J Antimicrob Chemother 69: 2785-96 | PubMed |
| Hebecker B, Naglik JR, Hube B, Jacobsen ID | 2014 | Pathogenicity mechanisms and host response during oral Candida albicans infections. | Expert Rev Anti Infect Ther 12: 867-79 | PubMed |
| Wartenberg A, Linde J, Martin R, Schreiner M, Horn F, Jacobsen ID, Jenull S, Wolf T, Kuchler K, Guthke R, Kurzai O, Forche A, d'Enfert C, Brunke S, Hube B | 2014 | Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. | PLoS Genet 10: e1004824 | PubMed |
| Schwartze VU, Santiago AL, Jacobsen ID, Voigt K | 2014 | The pathogenic potential of the Lichtheimia genus revisited: Lichtheimia brasiliensis is a novel, non-pathogenic species. | Mycoses 57 Suppl 3: 128-31 | PubMed |