FungiNet
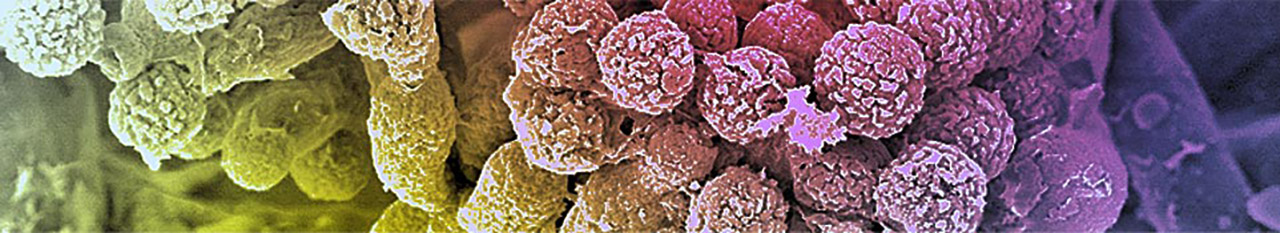
The incidence of life-threatening infections due to opportunistic fungal pathogens has drastically increased over the past two decades. This increase is associated with excessive mortality and directly related to a growing number of patients at risk. Despite this, the clinical diagnosis of these infections is still difficult and often too late for diagnostics-guided patient management. Options for antifungal therapy are limited. The polymorphic yeast Candida albicans and the filamentous fungus Aspergillus fumigatus are by far the most important causes of invasive mycoses in Europe. Scientists from two major sites in Germany, Jena and Würzburg, with a research focus on human-pathogenic fungi have joined forces in the CRC/TR FungiNet. Both sites run excellence graduate schools, which organize the training of doctoral and postdoctoral researchers of FungiNet.
58th Scientific Conference of the German speaking Mycological Society (DMykG) e.V. together with the CRC/Transregio FungiNet
The abstract submission for #MYK2024 is open! Don't miss the opportunity to share your groundbreaking research in Jena!
Deadline: 29 April 2024!
The aims of FungiNet are
(1) to identify pathogenicity determinants of C. albicans, A. fumigatus and the emerging fungus Lichtheimia corymbifera,
(2) to investigate the specific roles of epithelial barriers, the mechanisms of the innate immunity and contributions of the adaptive immune system to the pathogenesis of fungal infections,
(3) to identify common principles of fungal pathogenesis and
(4) to use this information for the development of new therapeutic approaches.
FungiNet has also included a systems biology-based approach as a third dimension. This has led to the generation of virtual infection models, which allow predictions that can be tested experimentally. In the first funding period, discoveries have been made that significantly enhanced our understanding of fungal pathogenicity. For instance, we discovered the first example of a peptide toxin from a human pathogenic fungus: Candidalysin from C. albicans. The CRC/TR integrates two university hospitals that treat the major patients groups who are at high risk for fungal infections: Intensive care patients (Jena) and haematology-oncology patients (Würzburg). Based on our results it has been possible to carry out genetic risk profiling of haematological patients. With continuation of the CRC/TR, the virulence determinants and receptors we discovered will be studied in further detail. Furthermore, our future studies will address the additional levels of complexity introduced by including the interaction of pathogens with tissue and the impact of microbiomes on fungal pathogenesis. In addition to advancing basic research, in the long term we aim to deliver the development of novel diagnostics and therapies through the identification of biomarkers, the development of immune cell therapy or the identification of novel targets for antimycotic therapy.