Project C6
Secreted fungal proteins in immune evasion and pathogenicity
The fungal pathogens C. albicans and A. fumigatus secret immune evasion proteins to control the immune response of the human host. In project C6, we characterize the role of secreted fungal proteins in immune evasion. Both fungal pathogens use related proteins like Pra1 (C. albicans) and Aspf2 (the Pra1 homolog of A. fumigatus) to bind host regulators and plasminogen. Plasminogen attached to either the purified proteins or to the fungal pathogens is accessible for activators and can be converted to the active protease plasmin. Active plasmin then damages host endothelial and epithelial cells and induces cell retraction. Thus, the subcellular matrix is exposed and further degradation of matrix components is induced. Such local tissue damage ultimately helps the pathogenic microbe to cross host tissue barriers and to disseminate into deeper tissue layers.
Several of the secreted fungal proteins are fungal moonlighting proteins. Examples for such multifunctional fungal immune evasion proteins are Tef-1 (Translations elongation factor 1), and Pra1 (the pH regulated antigen1) that both contribute to fungal pathogenicity. Both C. albicans moonlighting proteins mediate fungal immune escape by inhibiting both innate and adaptive immune reaction of the human host. We have generated monoclonal antibodies (mAbs) against these immune evasion proteins. In the next funding period, we will continue the in vitro studies and extend the analyses to fungal immune escape in mice. Moreover, we will evaluate our anti-fungal mAbs in mouse models of C. albicans infections.
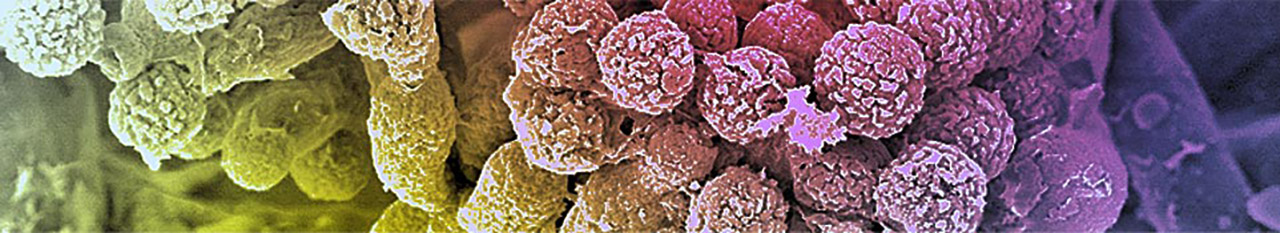
Human endothelial cells (HUVEC)
HUVEC cells + C. albicans with plasmin attached

C. albicans having the active protease plasmin attached to the surface damages human endothelial cells.
(A) Intact human endothelial cells (HUVEC) from a cell monolayer. (B) HUVEC cells challenged with C. albicans with the active protease on the surface are damaged. The cell retract and the sub endothelial matrix becomes exposed.
(C) C. albicans (arrow) attached to the surface of human cells.
(D) Enlarged view
| Author | Year | Title | Journal | Links |
|---|---|---|---|---|
| Halder LD, Babych S, Palme DI, Mansouri-Ghahnavieh E, Ivanov L, Ashonibare V, Langenhorst D, Prusty B, Rambach G, Wich M, Trinks N, Blango MG, Kornitzer D, Terpitz U, Speth C, Jungnickel B, Beyersdorf N, Zipfel PF, Brakhage AA, Skerka C | 2022 | Candida albicans induces cross-kingdom miRNA trafficking in human monocytes to promote fungal growth. | mBio 13(1): e0356321 | mBio |
| Ickrath P, Sprügel L, Beyersdorf N, Scherzad A, Hagen R, Hackenberg S | 2021 | Detection of Candida albicans-specific CD4+ and CD8+ T cells in the blood and nasal mucosa of patients with chronic Rhinosinusitis. | J Fungi (Basel) 7: 403 | J Fungi |
| Shopova IA, Belyaev I, Dasari P, Jahreis S, Stroe MC, Cseresnyés Z, Zimmermann AK, Medyukhina A, Svensson CM, Krüger T, Szeifert V, Nietzsche S, Conrad T, Blango MG, Kniemeyer O, Lilienfeld-Toal Mv, Zipfel PF, Ligeti E, Figge MT, Brakhage AA | 2020 | Human neutrophils produce antifungal extracellular vesicles against Aspergillus fumigatus. | mBio 11: e00596-20 | PubMed |
| Halder LD, Jo EAH, Hasan MZ, Ferreira-Gomes M, Krüger T, Westermann M, Palme DI, Rambach G, Beyersdorf N, Speth C, Jacobsen ID, Kniemeyer O, Jungnickel B, Zipfel PF, Skerka C | 2020 | Immune modulation by complement receptor 3 dependent human monocyte TGF-β1-transporting vesicles. | Nat Commun 11: 2331 | PubMed |
| Tille A, Lehnert T, Zipfel PF, Figge MT | 2020 | Quantification of factor H mediated self versus non-self discrimination by mathematical modeling. | Front Immunol 11: 1911 | Frontiers |
| Haack S, Baiker S, Schlegel J, Sauer M, Sparwasser T, Langenhorst D, Beyersdorf N | 2020 | Superagonistic CD28 stimulation induces Interferon y release from mouse T Helper 1 cells in vitro and in vivo. | Eur J Immunol [Epub ahead of print] | PubMed |
| Irmscher S, Brix S, Zipfel SLH, Halder LD, Mutlutürk S, Wulf S, Girdauskasm E, Reichenspurner H, Stahl RAK, Jungnickel B, Wiech T, Zipfel PF, Skerka C | 2019 | Serum FHR1 binding to necrotic-type cells activates monocytic inflammasome and marks necrosic sites in vasculopathies. | Nat Commun 10: 2961 | PubMed |
| Breitenbach T, Liang C, Beyersdorf N, Dandekar T | 2019 | Analyzing pharmacological intervention points: A method to calculate external stimuli to switch between steady states in regulatory networks. | PLoS Comput Biol 15: e1007075 | PubMed |
| Klaile E, Müller MM, Brehme S, Zubiria-Barrera C, Klassert TE, Stock M, Durotin A, Nguyen TD, Feer S, Singer BB, Zipfel PF, Rudolphi S, Jacobsen ID, Slevogt H | 2019 | Unaltered fungal burden and lethality in human CEACAM1-transgenic mice during Candida albicans dissemination and systemic infection. | Front Microbiol 10: 2703 | PubMed |
| Dasari P, Koleci N, Shopova I, Wartenberg D, Beyersdorf N, Dietrich S, Sahagún-Ruiz A, Figge MT, Skerka C, Brakhage AA, Zipfel PF | 2019 | Enolase from Aspergillus fumigatus is a moonlighting and immune evasion protein that binds the human plasma complement protein factors H, FHL-1, C4BP, and Plasminogen. | Front Immunol 10: 2573 | PubMed |
| Lang SN, Germrodt S, Glock C, Skerka C, Zipfel PF, Schuster S | 2019 | Molecular crypsis by pathogenic fungi using human factor H. A numerical model. | PLoS One 14: e0212187 | PubMed |
| Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, Bontha SV, Srikakulapu P, Beer M, Megens RTA, Steffens S, Hildner M, Halder LD, Eckstein HH, Pelisek J, Herms J, Roeber S, Arzberger T, Borodovsky A, Habenicht L, Binder CJ, Weber C, Zipfel PF, Skerka C, Habenicht AJR | 2019 | ApoE attenuates unresolvable inflammation by complex formation with activated C1q. | Nat Med 25: 496-506 | PubMed |
| Meinel C, Spartà G, Dahse H-M, Hörhold F, König R, Westermann M, Cseresnyés Z, Coldewey SM, Figge MT, Hammerschmidt S, Skerka C, Zipfel PF | 2018 | Streptococcus pneumoniae from patients with hemolytic uremic syndrome binds human plasminogen via the surface protein PspC and uses plasmin to damage human endothelial cells. | J Infect Dis 217: 358-70 | PubMed |
| Irmscher S, Döring N, Halder LD, Jo EAH, Kopka I, Dunker C, Jacobsen ID, Luo S, Slevogt H, Lorkowski S, Beyersdorf N, Zipfel PF, Skerka C | 2018 | Kallikrein cleaves C3 and activates complement. | J Innate Immun 10: 94-105 | PubMed |
| Hummert S, Glock C, Lang SN, Hummert C, Skerka C, Zipfel PF, Germerodt S, Schuster S | 2018 | Playing ’hide-and-seek’ with factor H: game-theoretical analysis of a single nucleotide polymorphism. | J R Soc Interface 15: 20170963 | PubMed |
| Dasari P, Shopova IA, Stroe M, Wartenberg D, Dahse HM, Beyersdorf N, Hortschansky P, Dietrich S, Cseresnyés Z, Figge MT, Westermann M, Skerka C, Brakhage AA, Zipfel PF | 2018 | Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. | Front Immunol 9: 1635 | Frontiers |
| Luo S, Dasari P, Reiher N, Hartmann A, Jacksch S, Wende E, Barz D, Niemiec MJ, Jacobsen I, Beyersdorf N, Hünig T, Klos A, Skerka C, Zipfel PF | 2018 | The secreted Candida albicans protein Pra1 disrupts host defense by broadly targeting and blocking complement C3 and C3 activation fragments. | Mol Immunol 93: 266-77 | PubMed |
| Halder LD, Abdelfatah MA, Jo E, Jacobsen ID, Westermann M, Beyersdorf N, Lorkowski S, Zipfel PF, Skerka C | 2017 | Factor H binds to extracellular DNA traps released from human blood monocytes in response to Candida albicans. | Front Immunol 7: 671 | PubMed |
| Klaile E, Müller M, Schäfer MR, Feer S, Heyl K, Stock M, Klassert T, Zipfel P, Singer P | 2017 | Binding of Candida albicans to human CEACAM1 and CEACAM6 modulates the inflammatory response of intestinal epithelial cells. | mBio 8: e02142-16 | PubMed |
| Bergfeld A, Dasari P, Werner S, Hughes TR, Song WC, Hortschansky P, Brakhage AA, Hünig T, Zipfel PF, Beyersdorf N | 2017 | Direct binding of the pH-regulated protein 1 (Pra1) from Candida albicans inhibits cytokine secretion by mouse CD4+ T cells. | Front Microbiol 8: 844 | PubMed |
| Pollmächer J, Timme S, Schuster S, Brakhage AA, Zipfel PF, Figge MT | 2016 | Deciphering the counterplay of Aspergillus fumigatus infection and host inflammation by evolutionary games on graphs. | Sci Rep 6: 27807 | PubMed |
| Buhlmann D, Eberhardt HU, Medyukhina A, Prodinger WM, Figge MT, Zipfel PF, Skerka C | 2016 | Complement factor H related protein 3 (FHR3) blocks C3d-mediated co-activation of human B cells. | J Immunol 197: 620-9 | PubMed |
| Berges C, Kerkau T, Werner S, Wolf N, Winter N, Hünig T, Einsele H, Topp MS, Beyersdorf N | 2016 | Hsp90 inhibition ameliorates CD4+ T cell-mediated acute graft versus host disease in mice. | Immun Inflamm Dis 4: 463-73 | PubMed |
| Dühring S, Germerodt S, Skerka C, Zipfel PF, Dandekar T, Schuster S | 2015 | Host-pathogen interactions between the human innate immune system and Candida albicans - understanding and modeling defense and evasion strategies. | Front Microbiol 6: 625 | Frontiers |
| Luo S, Hipler UC, Münzberg C, Skerka C, Zipfel PF | 2015 | Sequence variations and protein expression levels of the two immune evasion proteins Gpm1 and Pra1 influence virulence of clinical Candida albicans isolates. | PLoS One 10: e0113192 | PubMed |
| Zipfel PF, Skerka C | 2014 | Staphylococcus aureus: the multi headed hydra resists and controls human complement response in multiple ways. | Int J Med Microbio 304: 188-94 | PubMed |